DDI Functional Genomics Team Has Knocked-out GCGR in Zebrafish
Published on 03/07/2023
The Glucagon receptor, GCGR, is a membranous receptor that plays an essential role in regulating glycemia. The main action of glucagon is to stimulate hepatic glucose production by increasing glycogenolysis and gluconeogenesis. Furthermore, glucagon has positive inotropic effects on the heart, increases lipolysis in the adipose tissue, acts as a satiety factor in the central nervous system, and has regulatory effects on insulin. Therefore, the GCGR plays a crucial role in maintaining glucose homeostasis and providing energy to the body. Dysregulation of GCGR signaling can lead to metabolic disorders such as diabetes and obesity. This dysregulation can be studied by inducing mutations in the GCGR gene.
Dr. Ashraf Al-Madhoun and his team are trying to unravel the function of a variant called rs1801483 that generates a missense mutation called Gly40Ser (G40S). Their preliminary results revealed that the minor allele frequency of the rs1801483 in the coding region of the GCGR gene is 3.7% in the Kuwaiti population (4.4% and 2.9% in 114 diabetic and 168 non-diabetic Kuwaiti subjects, respectively), compared to 0.1 to 1.9% in other populations. They also identified the G40S mutation in seven Kuwaiti families, whose affected members suffer from diabetes. Receptor binding studies using cultured cells expressing the G40S mutation showed that this mutation results in a receptor that binds glucagon with a 3-fold lower affinity compared to the wild-type receptor. However, the molecular and cellular mechanism that is mediated by this variant is unknown. This variant can be a target to treat diabetes by designing a specific glucagon-mimicking substrate.
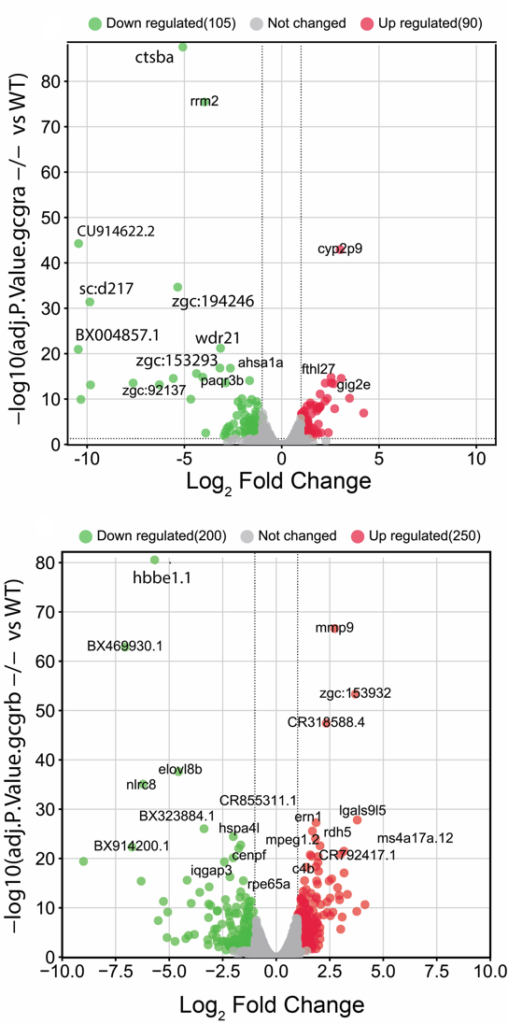
Zebrafish (Danio rerio) is a widely used model organism in genetics and developmental biology research due to its small size, transparency during embryonic development, and genetic similarity to humans. Zebrafish have approximately 26,000 genes in their genome. Studies have shown that zebrafish have a functional pancreas with both α- and β-cells producing and secreting glucagon and insulin, respectively, in response to changes in blood glucose levels. In fact, zebrafish have become a popular model organism for studying the regulation of glucose homeostasis and the development of metabolic diseases such as diabetes due to their genetic and physiological similarity to humans. Research on the production and secretion of glucagon in zebrafish can provide valuable insights into the mechanisms underlying glucose metabolism and the development of metabolic diseases in humans. Zebrafish have two copies of the GCGR gene, named GCGRa and GCGRb. Using CRISPR technology, Dr. Al Madhoun and his team have knocked out (KO) both genes separately. RNA sequencing analysis revealed differential target gene regulation between GCGRa and GCGRb, particularly in genes regulating fatty acid biosynthesis and oxidative phosphorylation pathways.
The percentage of gene homology between zebrafish and humans varies depending on the specific function of these genes and use this information to develop new treatments for human diseases. Many Zebrafish genes don’t have a known function; the Department of Genetics and Bioinformatics will be gene being compared. On average, it is estimated that zebrafish share approximately 70% of their genes with humans. By comparing the homology of zebrafish genes to creating a tool to predict the functional annotation of these genes.
The differential gene expression study of GCGR KO versus control is those of other organisms, researchers can gain insights into the evolutionary conservation and depicted in Figure 1 as a volcano plot. The highlighted genes are highly expressed. Green indicates down regulated genes, while red indicates increased genes.


