Early Macrophage response to obesity encompasses IRF5-regulated Mitochondrial Architecture Remodeling
Published on 29/12/2022
Adipose tissue macrophages (ATMs) are sentinel cells that respond to microenvironmental cues. In response to chronic energy imbalance in obesity, ATMs accumulate in adipose tissue and undergo polarization along a spectrum, in an attempt to restore homeostasis. Dr. Fawaz Alzaid’s group at INSERM (Paris, France) previously discovered that the transcription factor — Interferon Regulatory Factor 5 (IRF5) — is a critical mediator of the inflammatory polarization of ATM and plays a causal role in the onset of insulin resistance. Subsequently, research in the field of immunometabolism demonstrated that macrophages adapt their cellular metabolism in support of their inflammatory functions. Innovative genetic studies have also demonstrated that the IRF5 gain-of-function risk variants result in increased glycolytic flux in response to canonical toll-like receptor ligands.
With this knowledge, Dr. AlZaid hypothesized that IRF5-signaling in ATMs plays a parallel role in adapting cellular metabolism in ATMs. His team worked with models of short-term and long-term caloric excess and demonstrated that oxidative respiration of ATMs is repressed early in the course of obesity, i.e., at a stage where glucose intolerance occurs, however, prior to the onset of insulin resistance. Oxidative respiration is typically associated with anti-inflammatory polarization, in contrast to IRF5-mediated inflammatory polarization. Repression of oxidative respiration is alleviated in mice with a myeloid-restricted deficiency of IRF5. Hyperoxidative ATMs in IRF5-deficiency allow macrophages to better handle lipotoxicity in the adipose microenvironment.
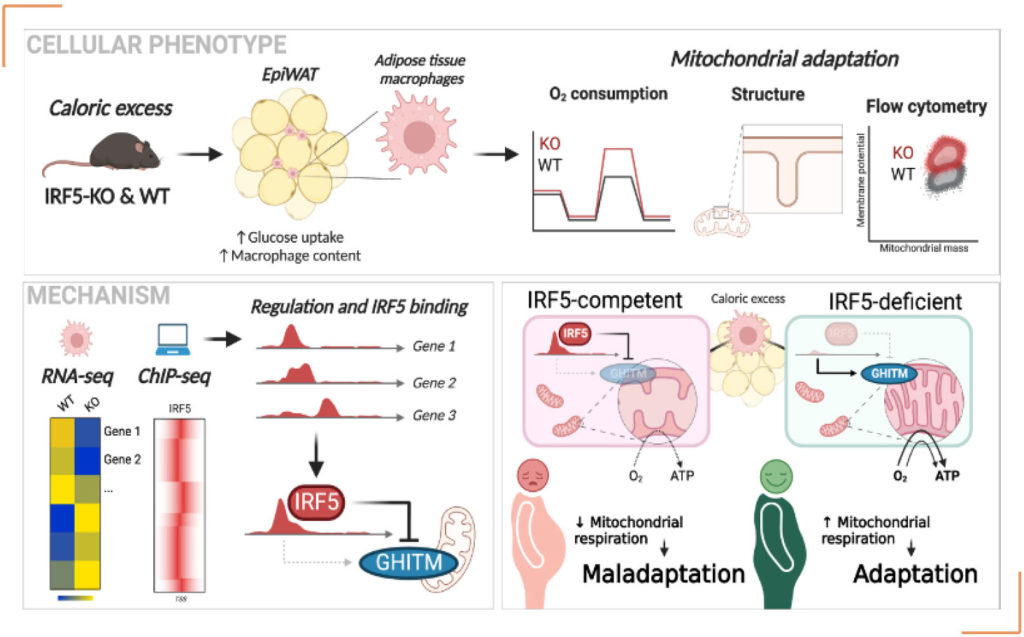
Dr. Alzaid said: “To decipher an underlying mechanism, we first demonstrated that these effects were cell autonomous, inducible, and reversible, being observed in ex-vivo differentiated bone marrow-derived macrophages. We next integrated in-house RNA-seq from multiple models with public ChIP-seq data to discover transcripts regulated in an IRF5- dependent manner. We found a target gene repressed by IRF5 that is involved in regulating mitochondrial structural components necessary for oxidative respiration. The gene coding for the Growth Hormone Inducible Transmembrane (GHITM) protein maintains mitochondrial cristae structures. These are finger-like projections that provide docking points for oxidative phosphorylation enzyme complexes in the mitochondria. In IRF5- deficiency, GHITM expression is derepressed, and as a result, cristae structures are well-developed allowing a higher surface area for oxidative respiration. We demonstrated that the binding mechanism and mitochondrial functional adaptation are conserved in monocytes and ATMs from patients with type-2 diabetes and obesity.” This work was carried out by Dr. Fawaz Alzaid’s group at the IMMEDIAB team at INEM (Paris, France) and was published in Nature communications.


